Problem introduction:
These days we live an unprecedented era, where gifts we used to take for free from Mother Earth , can run out in the blink of an eye. By the gifts it’s not meant only the crude oil or building materials being mined in a vast amounts… As humans we tend to overlook the things which are so natural for us that we don’t even think of the possibility that they could be missed one day… Have you ever imagined what would we do if all the drinking water resources suddenly disappeared? The biggest drinking water resources are glaciers. And glaciers melt… More and more… It’s just the matter of time when they will be lost completely. So what can we do?
If we cannot harvest some resource/material directly there is still an alternative way – to produce it. The same applies for the drinking water. The know-how of turning the polluted water into a potable form is needed… quite a lot.
Yes, almost everyone knows that world possesses plenty of seawater (actually, it’s even more than plenty). And that we can turn into drinking water. By boiling it and then condensing we can get distilled water out of which we can after the proper addition of minerals prepare the potable water. The problem lies in the fact that the amount of energy which we need for the water to change its state from liquid to gas is big (because of the water characteristics – concretely heat capacity). So for bigger amounts of water, we would need extreme amount of energy… and time. And both these are very scarce, especially these days.
That’s why it is essential to work on energetically less consuming water purification methods. One of the promising water purification methods are membrane processes. They use thin, porous layer (membrane) to separate different particles from aqueous solutions. Special position in terms of water transport through membranes have so called aquaporin proteins. These proteins selectively permit water… And nothing else. That’s why they are applied in these membranes.
Using them water is separated from other substances dissolved (or non-dissolved) in it by passing through this aquaporin membrane which doesn’t allow the substances to go through it. Simply said, the only tolerated passenger is water, no other travelers are allowed.
Now the only need is to persuade the water to flow through the membrane in an energetically efficient way. Because the truth is that it doesn’t want to… What’s more, it wants to flow reversely. Water naturally wants to ride from a less concentrated area to a more concentrated one. This natural process is called osmosis.
Once more… What do we need for passing water through the membrane? Quite unsurprisingly, as for distillation, we need energy. However it’s much less, it is still needed.
The reason why it is better solution is because we use a natural process which is present in every living cell. Nature has built this process to allow the cells to exchange water between each other. And that is also the reason why is this process energetically optimal – every natural phenomenon is optimised and so the osmosis is.
If we were successful in beating this natural osmotic force we could then get what we need. Now the question is how… Well, very easily (in terms of application, energetically it is not that easy). Just by pushing the water using hydraulic piston. This way we create so called hydraulic force direction of which is opposite to the osmotic force. And when we make it higher than the osmotic force, the flow of the water will change. And we will get what we need. This method which uses hydraulic pressure to “persuade” the water to flow through the membrane is called reverse osmosis (because the transport of the water is in the opposite direction compared to osmosis). It is used to separate diluted ions. If we needed to separate water from even smaller particles we could then use nanofiltration where membranes with smaller pours are used.
Reverse osmosis is quite well known and used for various purposes. It is also used in water purification devices which turn the non-potable water to potable. But sometimes it cannot be applied so easily. For example, when we have too contaminated water and we apply reverse osmosis on it, the membrane fouls, i.e. impurities stuck on the membrane surface and so they dysfunction it. For the case of pre-treatment of this feed water we need to use some other technology using which we separate various impurities from water. When we extract the water to some other, cleaner solution we can consequently get it using reverse osmosis easier. We can reach this using so called forward osmosis process.
As already mentioned, water naturally transports from less concentrated solutions to more concentrated to erase the concentration gap. And forward osmosis uses exactly that. It is based on attraction of the water from the feed to so called draw solution, which contains more concentrated solutions. Therefore they can osmotically attract water and this way separate it from (to membrane harmful) impurities. After that is then the pure water extracted from this diluted draw solution. The picture below explains it in an easier – more understandable way.
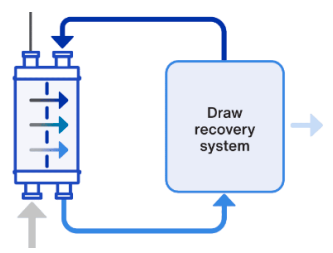
To ensure continuous functioning of this whole purification system we need to regenerate draw solution to its original concentration and put it back to the draw tank. Otherwise, it would lose its drawing – “water extracting” force – that would happen when the concentration of the diluted draw solution would reach the concentration of feed solution.
Membrane processes as the water purification methods are the field of study of Danish company named Aquaporin (name derived from above mentioned water-selective protein). And one of their researched membrane techniques is also forward osmosis. Our project (closer described below) has been developed in mutual collaboration with this company. Content of their work is very well described in the video bellow.
Process modelling:
These are the proofs of why it is worth to talk about the practical application of forward osmosis and work on its model design. Because by modelling it we can get to know the system – how it behaves, what is its dynamics like and how given inputs influence it. Thanks to it we can test various situations, alternative setups , and find the best one – maximum water, minimum energy. So simply said we can test the system virtually – without the need of having real device. We just need to know its characteristics.
Now let’s move back to our task. Based on facts stated above we can create a scheme of the process.
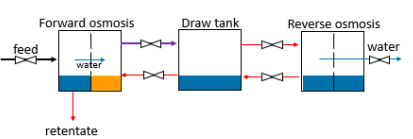
Feed is flowing through the forward osmosis membrane because it is attracted by the draw solution. So in the left part of the first compartment remains concentrated feed (retentate), in the right part is diluted draw solution – diluted because it is mixed with water which flows from the feed through the membrane. From draw tank flows draw solution to FO and RO compartments and back to it flows from FO its diluted form and from RO compartment its concentrated form. Finally, out of Reverse osmosis compartment flow the needed permeate – pure water. There’s one manipulated input (value of which we choose), that is a mass flow from FO membrane to draw tank, and two disturbance variables (values of which are given, we cannot change them), those are mass flow and concentration of the feed…
Every mathematical model is built on some physical laws and equations which describe relations between given physical quantities of the process. In our case we have constructed the model applying total mass balance and solute mass balance rules, interpretation of which is simply just: Change of the system’s hold-ups equals to the difference of inlet and outlet flows. It’s basically a law of the mass preservation.
This led us to creation of dynamic equations which we then simulated in Matlab environment. Firstly, we wanted to find out a steady state of the process. We used Matlab built-in functions (fsolve, ode45) to achieve this.
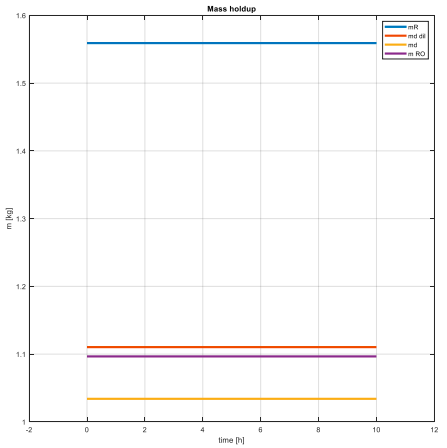
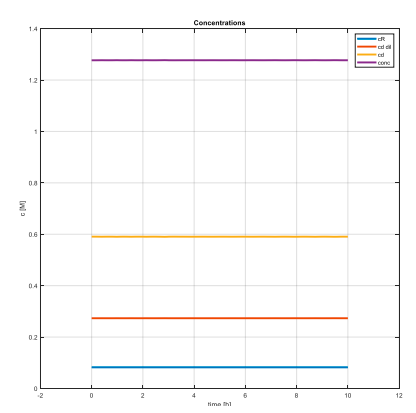
Graphs confirm that the steady states have been successfully achieved and also that their values are meaningful – concentration of feed must be smaller than concentration of a diluted draw solution, which in the same time must be smaller than the original draw solution’s concentration… And the highest one needs to be the concentration of solution flowing out of reverse osmosis membrane to draw solution. These conditions are simply derived from the principles of the forward osmosis process. That’s meant that for example the concentration of diluted draw solution necessarily needs to be higher than feed’s concentration, otherwise the water doesn’t flow through the FO membrane.
Secondly, we wanted to find out how the process behaves when we make a step change – that means we wanted to find its dynamics. To do that we changed value of given input variables and observed system’s response.
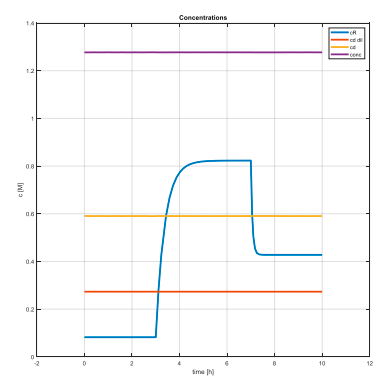
As we can see, these step changes have affected only one state – retentate concentration. The charts provide us a basic overlook on the process dynamics and states–inputs relations. That’s why it is important for us.
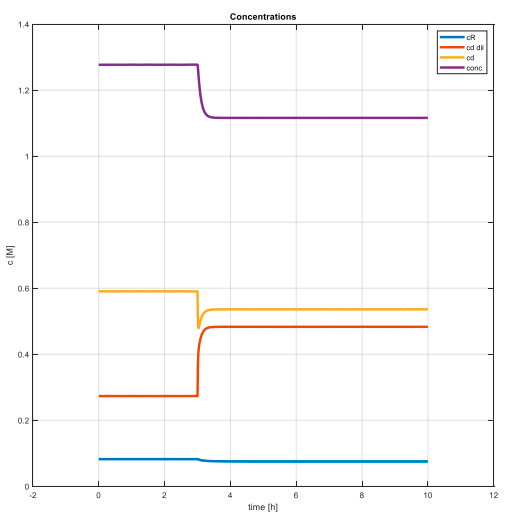
The charts provide us a basic overlook on the process dynamics and inputs-states relations. That’s why they are important for us.
Conclusion:
Aim of our work was to design a model of forward osmosis process, because it is crucial for understanding the system. Without it we can not move further – improve it, optimise,… That’s why it is crucial to have it. And why do we want to study, implement and develop such processes? Because once when the whole fresh water will be gone, they might save our lives.